
The number of highly potent drugs in OSD (Oral Solid Dosage) production has risen sharply in recent years. As a result, containment is becoming ever more important in the production of these materials. For those who work with highly active ingredients in OSD development, they cannot avoid the use of containment due to potential leaking materials and faulty cleaning, which can lead to lethal outcomes. Tightening regulations by the legislator and more complex manufacturing processes also underline the need for a high-quality containment solution.
What is a Potent Compound?
A compound is generally classified as potent if:
- It has an occupational exposure limit (OEL) of ≤ 10 μg/m3
- It has a daily therapeutic dose of ≤ 10 mg/day
- It has biological activity at 150 μg/kg body mass in humans
- It has high pharmacological selectivity and/or with potential to cause cancer, cell mutation, reproductive toxicity at low doses
1 μg = 0.000001 gram (1 millionth of a gram)
1 ng = 0.000000001 gram (1 billionth of a gram)
Definitions
Occupational Exposure Limit (OEL): At or below 10 µg/m3 of air as an 8h time-weighted average.
Short-term exposure limit (STEL): Is the acceptable exposure limit to a toxic or an irritant substance over a short period of time (time-weighted average), usually 15 minutes with a maximum of 4 peaks/day with a minimum of 1 hour between exposures.
The Acceptable Daily Intake (ADI): Is commonly defined as the maximum amount of a chemical to which a person can be exposed, daily over an extended period, usually without suffering a deleterious effect.
Putting it in perspective!
- A grain of table salt weighs approx. 58.5μg

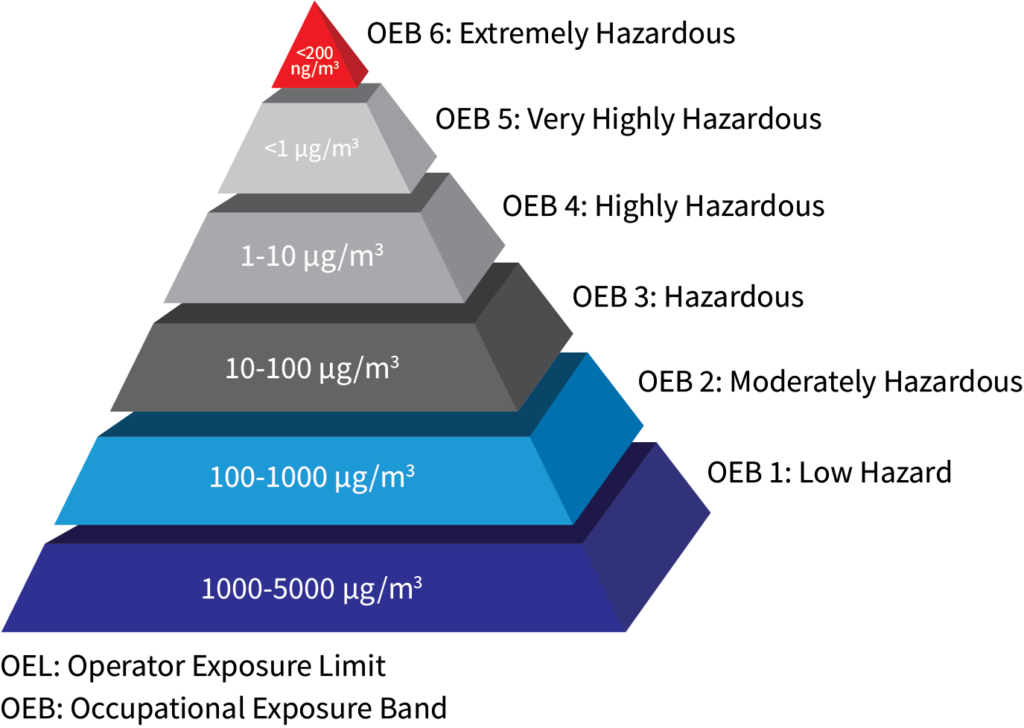
Classification of Potent Compounds
Occupational Exposure Band (OEB), also known as hazard banding, is a process intended to quickly and accurately assign chemicals into specific categories (bands), which correspond to a range of exposure concentrations designed to protect worker health. These bands are assigned based on a chemical’s toxicological potency and the adverse health effects associated with exposure to the chemical.
There are many different approaches and concepts for small scale and production scale when it comes to containment and it can all depend on the toxicity level of the active substance to be processed.
Protection and Containment Methods
Operator Protection via PPE:
OEB 1: 1000-5000 μg/m3 compounds are considered non-toxic.
- Protection Focus: Operator and PPE (personal protective equipment)
- Typical PPE includes cGMP gowning + gloves + safety glasses
OEB 2: 100-1000 μg/m3 compounds are typically considered almost non-toxic.
- Protection Focus: Operator and PPE (personal protective equipment)
- Typical PPE includes cGMP gowning + gloves + safety glasses + disposable dust mask
OEB 3: 10-100 μg/m3 compounds are typically considered slightly toxic.
- Protection Focus: Operator and PPE (personal protective equipment) with shift heavily toward equipment containment
- Typical PPE includes cGMP gowning + gloves + safety glasses + half-face respirators
OEB 4: 1-10 μg/m3 compounds are typically considered potent.
- Protection Focus: Equipment and material handling, but PPE may be used for existing equipment or where limited usage makes PPE more practical
- Typical PPE includes PAPR (powered air purifying respirators)
OEB 5: <1 μg/m3 compounds are considered highly potent
- Protection Focus: Equipment containment and closed methods of material handling
- Typical operator protection would require full air fed suits

Operator Protection via Containment:
OEB 3: Downflow booths are frequently employed to achieve OEB 3 levels of containment
- Engineered control that achieves containment by air entrainment
- Unidirectional airflow entrains dust particles, preventing them from becoming airborne
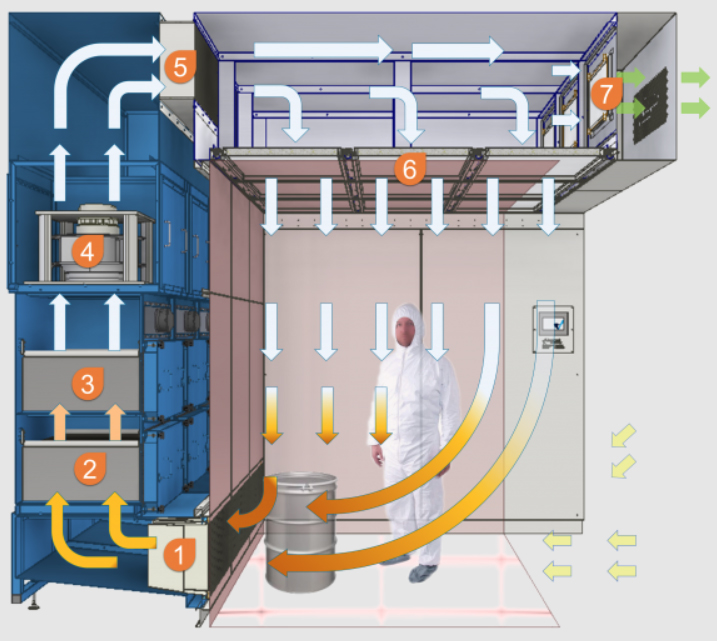

OEB 4: With the addition of a containment screen, OEB 4 containment levels can be achieved

OEB 4/5: At the < 10 ug/m3 containment level, closed transfer of materials and equipment isolation becomes required in most cases. Closed loading and unloading of materials are commonly achieved with a split butterfly valve (SBV) or via isolator technology. Split Butterfly Valves consists of two halves, a passive half, and an active half. The active half remains fixed while the passive half is portable. When docked in place and locked together, the two halves open together, leaving clean surfaces between them.


For optimal handling and easy and fast cleaning, bags and or bottle systems are often used at loading and sampling points.


Split Butterfly Valves are also used with an IBC for charging and discharging of materials or tablets.
Isolators: Flexible or Rigid
Flexible isolators do not require long cleaning and costly validations like traditional containment. Single-use also eliminates the risk for cross-contamination by providing an economically feasible and technical solution for most production facilities. The life of a single-use flexible isolator is more robust, as flexible isolators can be used multiple times while effectively maintaining risk elimination.
- Pros: Faster manufacturing, lower relative cost, easier to adapted to existing equipment

Rigid isolators are primarily 316L stainless steel with laminated safety glass viewing window. Glove ports are mounted on the window where glove sleeves are mounted. Inlet and outlet HEPA filters with positive or negative blowers, utility panels and ports, like an RTP (Rapid Transfer Port) for introducing or removing items to or from the isolator can also be provided. These isolators are more expensive than flexible isolators. However, there are many advantages to using a rigid isolator.
- Pros: Highly customizable, lower ongoing operating costs, no single-use waste generated


Environmental and Operator Protection
Explosive containment: click here to lean more!
Material Handling
Pharmaceutical manufacturers must take every precaution to ensure the safety of their products for the protection of employees. Safe handling practices are also essential for maintaining compliance with FDA regulations. The choice of material handling equipment is one of the most critical decisions that pharma companies face regarding the prevention of contamination during processing. Material handling comes in many different methods for all types of equipment in the pharmaceutical industry ranging from:
- Intermediate Bulk Containers (IBCs)
- IBC: Powders or Tablets
- IBC: Charge/Discharge: Gravity or Vacuum
- IBC: Washing System
- IBC: Post Hoist Lifts
- Dispensing/Filling
- Weighing and Dispensing Systems
- Drum/Bag Handling
- IBC Filling
- Contained Dispensing
- Portable Drum Tippers
- Blending/Mixing
- IBC: Blending Systems
- V-Shell, Double-Cone or Offset Cone Blending
- Laboratory Scale Blending Systems
- Lifts
- Fluid Bed Bowl Inverter Lifts
- In-line Milling/Sieving
- Drum Lifts
- Conveying Systems
- Pneumatic Conveying Systems for Powders
While each play an important role, trying to find the right pharma equipment can be a lot like wandering through one of those big box stores. While you will find many options, it can be difficult to judge their quality and reliability. Our expertise resolves this dilemma by allowing you to have a conversation with our specialists who understand the pharmaceutical industry and can make well-informed recommendations that can save you time and hassles.
Safe Cleaning

The cleaning concept must at least ensure that all materials are bound before the system is opened. Spray nozzles are located within the isolator for cleaning 95% of the machine while wash wands can be placed inside as well to clean behind shadow areas. The water is then collected separately. Minimum requirements are relatively easy to implement but are far from sufficient for larger plants and high requirements. Cleaning is carried out by one of three ways:
- Wet-In-Place: Commonly utilized method to speed up the cleaning process. Wetting down and removing most surface material prior to further cleaning. Wet-in-place is also used to wet parts that would have come into contact with powder; reducing the risk of airborne dusts when the equipment is removed for cleaning.
- Wash-In-Place (WIP): Implies a semi or fully automated cleaning with either undefined result of cleaning or with the result that the system is not yet clean according to GMP-requirements.
- Clean-In-Place (CIP): Set of procedures conducted to properly clean all or part of a process system as it sits in place, without removing or disassembling of equipment to accommodate the cleaning. Freund-Vector’s EcoClean™ (CIP) System is the best in the industry because of the following:
- High Pressure / Low Volume Skid
- Higher pressure provides faster cleaning
- Lower water usage eliminates need for tanks
- Lower water usage reduces operating costs
- Lower water usage means less wastewater

Using a riboflavin test plays an important part in proving cleanability. The test involves coating all surfaces to be cleaned with a riboflavin (vitamin B2) solution that glows under a black light or UV-A light and sprayed throughout the equipment that needs to be tested for cleanability.
Once a coverage test rinse is complete, critical cleaning areas are visually inspected using a black light and telescoping mirror to ensure the riboflavin solution has been completely removed. No visible fluorescence verifies complete spray coverage. If there is a residue showing under the black light, the design team can determine where the spray device coverage has not reached.

Large Scale Equipment Containment
Using an isolator on large scale production equipment is not the best solution, Freund-Vector can help you with a custom engineered design where the equipment is created around your process, the room and many more factors.

Containment Verification
The process of validating and defining the containment device according to the defined OEL level is known as surrogate monitoring. Surrogate monitoring is a stringent validation process to test whether the containment system is valid per the defined OEL category.

Methodology for Surrogate Monitoring:
- Validation Objective: To evaluate the effectiveness of containment solutions with the Active Pharma Ingredients (APIs) for its intended purpose, as per the ISPE guidelines.
- The evaluation performed using surrogate material like lactose, naproxen sodium, or paracetamol due to their physical properties, low toxic levels, samples even with low concentration can be quantified.
- The samples for iteration include background samples, personal breathing zone samples, area/static/emission samples, and swab samples.
- Air samples are collected at the normal operator breathing zone and multiple emission sources that might be released/ dispersed from the isolator.
- Surface swab samples to be collected to detect surface contamination.
- A certified industrial hygienist selects a location for area/static/emission samples and swab samples.
- Instruments need to be calibrated using a primary calibrator (before and after each sample) to test the accuracy of volume air passed through the sample.
- Other factors that could sway results are temperature and relative humidity that required monitoring throughout the process.
When selecting containment solutions for manufacturing a particular drug, it is essential to test the accuracy and effectiveness of equipment based on targeted performance. The result is validated through a Factory Acceptance Test (FAT) performed at a manufacturer’s site and later at the customer’s site called Site Acceptance Test (SAT).
Every Process Is Different
Since each process places various requirements on containment, a specific design is required, which is based on the size of the system, the space conditions, the toxicity of the products and the needs for product and operator protection. It is also important to consider the individual attitude of the operators and operators to the above topics to achieve an acceptable result for all sides involved.
So, the next time your project requirements a certain level of containment, think Freund-Vector, because we are a well-known supplier and expert of all-in-one containment solutions for both material handling and processing requirements.
