For the longest time, pharmaceuticals have been produced using a method known as “batch manufacturing,” which involves a multi-step, lengthy process that uses large-scale equipment. In recent years however manufacturing technology have prompted the pharmaceutical industry to consider moving away from batch manufacturing to a faster, more efficient process known as continuous manufacturing.
At Freund-Vector, we think that continuous manufacturing is increasingly meeting a new need in the marketplace. It takes a drug from raw materials to final product – seamlessly – with no need to shut down equipment or have any down time as the product is created. The poster represented below shows great results to this technology.
Using Continuous Granulation to Make Robust and High Drug Load APAP Granules
Since most pharmaceutical ingredients (API) have low density and poor flowability, wet granulation is practiced extensively for ease of handling. Wet granulation can be achieved by means of high shear mixers, fluid beds and twin-screw granulators. The former two methods are commonly used batch processes with long processing times. In addition, scale-up trials can be expensive, time consuming, and complicated. Twin-screw granulation offers the advantages of being easily adaptable into a continuous manufacturing system and elimination of scale up studies.
The Granuformer® (Gf-215) is an innovative new system that can perform granulation and drying continuously. The process begins with kneading and blending the raw materials, which feds a twin-screw extruder and vertical granulator then ends with a spiral dryer where the granules are dried by heated air and then collected by a cyclone with results in tighter control of final granule size and moisture content. The system leads to faster granule development, drying and 1-2 min testing times. The Granuformer® has less material waste before attaining steady state and after the operation of the system is shut down. Compared to conventional batched based production, this revolutionary device shows marked improvement in quality, productivity and achieves reduction in defection and development cost.

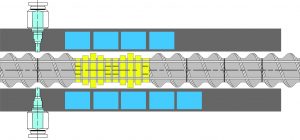
This study explored the advantages of continuous granulation to make high load acetaminophen (APAP) granules and studied the effect of polymer choice and process parameters on making robust granules and tablets.
METHODS
Table 1: Formulations:
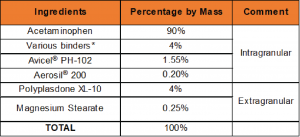
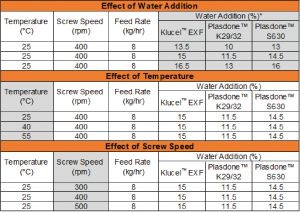
Table 2: Experimental Design:
Conclusions:
Continuous granulation is capable of making stronger, yet fast-dissolving tablets. All polymers yielded robust formulations and there was minimum effect of process variables on tablet hardness. The Gf-215 provides high mixing intensity, improved tablet strength and robustness.
To read or download the poster, Please click here. If you are interested in learning more about how Freund-Vector’s Granuformer®(Gf-215) can assist with your continuous granulation and drying needs, please send email to sales@freund-vector.com
Granuformer® is a registered trademark in the United States, Europe, and Japan
